Tropical disease caused by plasmodium faciparum study made in 2017 by Dr Rostand Idriss Tchana
OVERVIEW OF MALARIA
In this chapter, we will talk about malaria, the world situation regarding this pathology, the case of the Democratic Republic of Congo, the agent responsible for it, the medical diagnosis of the disease and finally the current methods taken in terms of treatment.
I. REMINDERS ON MALARIA
Malaria is a parasitic disease caused by a blood protozoan of the type Plasmodium (Lariviere, 1987; AECP, 2007; Hunt, 2007). This acute or chronic parasitaemia is caused by a protozoan of the type Plasmodium which is transmitted to humans by a vector agent, the anopheles. Transmission to humans is through the bite of a musquito, the female Anopheles (Fattorusso et al. 2004; Yuda et al., 2004; Quevauvilliers et al., 2007; Quevauvilliers et al., 2009; Aubry et al., 2015 ).
This pathology is characterized by several symptoms, including: cyclic febrile states, shivers, nausea, emesis, sudation, headaches, diarrhea, stiffness, splenomegaly, anemia, jaundice and several other symptoms that may be individual. The disease is complicated by the passage from a simple state to a severe state, often accompanied by severe anemia, renal failure, coma, high fevers, central nervous system involvement, respiratory problems often leading to death. Those most affected are children under 5 years old, non-immune adults and pregnant women (WHO, 2015).
II. MALARIA AROUND THE WORLD
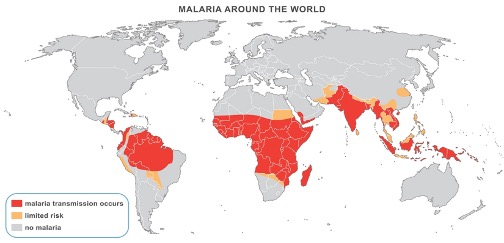
In 2016, 91 countries were listed as malaria-endemic territories. The number of malaria cases was estimated at 214 million and the number of associated deaths at 437,000. In Africa, access to tools to prevent and treat the disease is still very difficult (WHO, World Malaria Report 2016).
Tropical or sub-tropical Africa accounts for more than 80% of cases and 90% of mortality (Greenwood et al.,2005, Steketee et al., 1996; Fidock et al.,2004). Moreover, if we consider the indirect effects of malaria and its manifestations correlated with other diseases, the number of deaths would be even greater than that which is reported each year (Rogers et al., 2002; Hay et al., 2004; Christopher et al., 2012). On the highly exposed African continent (Figure 1), malaria is the leading cause of morbidity and mortality, contributes greatly to underdevelopment and poverty, and thus establishes a great barrier to socioeconomic development. It has been estimated that malaria costs Africa more than US$12 billion each year (Dorsey et al., 2000; Bloland et al., 2002).
Since the early 1990s, with a global awareness of the management of malaria and other neglected diseases, and with the aim of reducing malaria mortality and morbidity, the founding organizations of Roll Back Malaria (RBM) and the Abuja Declaration Action Plan in Nigeria have developed new approaches to reduce malaria, tuberculosis, and HIV/AIDS worldwide (Ridley, 2001; 2002).
In the last ten years, Africa is threatened by the decline of its health system, lack of infrastructures, climate change, instability, permanent atmosphere of insecurity, and manifestations of other serious pathologies (HIV/AIDS). All of these factors have a negative impact on immunity and lead to the development of malaria in areas with temperatures above 18°C and high humidity (where P. falciparum is still involved in more than 90% of cases) (Greenwood et al., 2005).
III. EPIDEMIOLOGY :
Malaria is a major public health problem in endemic countries, particularly sub-Saharan African countries with tropical and sub-tropical climates. Temperature, humidity, wind, and rainfall are factors that influence the complete cycle of Plasmodium and its transmission from one person to another (Jonathan, 2006; Pascual, 2006). Transmission can also occur in the absence of mosquitoes, for example in the case of pregnant women with congenital transmission via the placenta (Menendez, 1994).
IV. CARRIERS
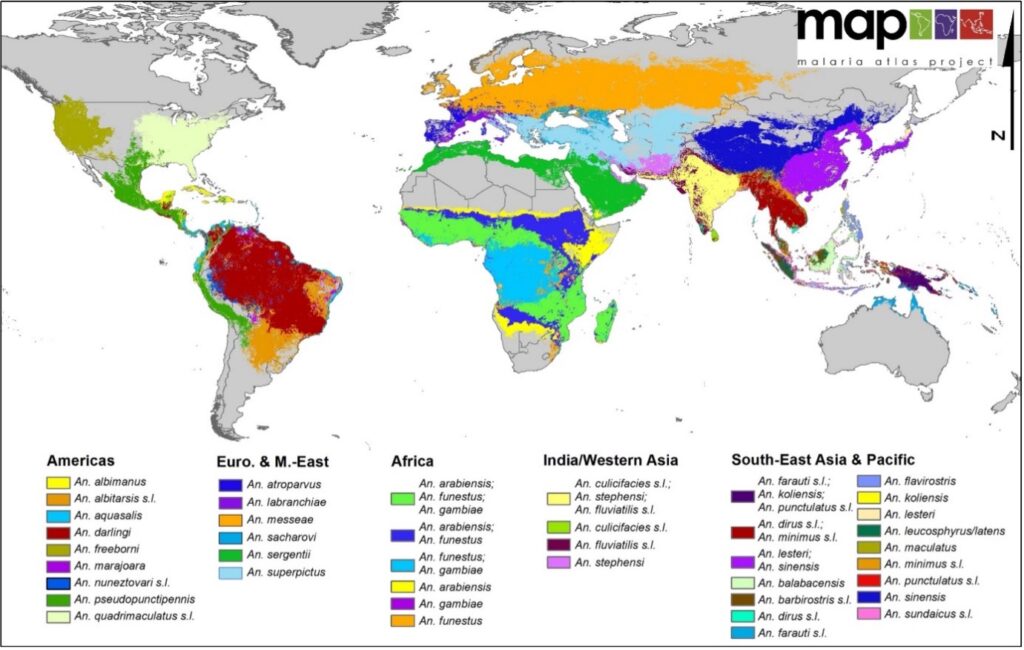
The vector of the disease is a female insect of the Diptera order, of the Culicidae family, subfamily Anophelinea and Anopheles gender (Figure 2). There are about 3500 different species of mosquitoes grouped in 41 genii. Among these, the genus Anopheles has at least 430 species, of which 30-40 are known to transmit disease to humans (CDC) (Figure 3).
The life expectancy of an Anopheles is 2-3 weeks. The Anopheles that survive for a long time allow the accomplishment of the Plasmodium cycle (e.g. 10 days at 25°C for P. falciparum).

Figure 2. Anopheles Arabiensis during its blood meal (Source: www.vectorbase.org, 2017)
V. PLASMODIUM
The Plasmodium responsible for malaria is an intracellular, amoeboid protozoan parasite that colonizes red blood cells and produces a pigment. During the life cycle, the parasite alternates between asexual reproduction (schizogony) in the vertebrate host and sexual reproduction (sporogony) in the invertebrate host, the Anopheles (Molez, 1993).
Plasmodium is an amoeboid intracellular parasite (Fig.4), of the phylum Sporozoa or Apicomplexa. It belongs to the order Heamosporidea, the latter being composed of only one family: Plasmodiidae; various genus are described, the genus Plasmodium having particular characteristics, such as the male and female gametocyte stage with different morphology.
VI. Taxonomy :
Plasmodium falciparum belongs to the domain, Eukaryota of the kingdom Chromalveolata, of the division Alveolata, it is included in the phylum Apicomplexa, of the class Aconoidasida, in the Order Haemosporida, of the family Plasmodiidae.
There are over 136 species of plasmodium (Marchiafava and Celli, 1895), the most important of which are the parasites of humans and rodents.
Five species are pathogenic to humans: P. vivax, P. ovale, P. malariae and P. falciparum; it has recently been confirmed that P. knowlesi (a parasite of primates in Southeast Asia, of the subgenus Plasmodium) is also capable of infecting humans (Singh et al., 2004). (Singh et al., 2004; Cox- singh et al., 2008, Chitnis and Miller, 1994).
For our research, we have selected Plasmodium yoelii, a rodent pathogen that is the model of choice for the study and simulation for human malaria. Plasmodium yoelii is one of many types of rodent parasites. Studies have shown that this parasite has many similarities with human Plasmodium: similarities in structure, physiology and life cycle (Diggs et al., 1977). In spite of these similarities, small differences remain, such as a variation in certain surface proteins that allow the invasion of red blood cells. The cycle of Plasmodium yoelii involves two hosts: the rodent, the intermediate host, hosts the asexual or schizogonic multiplication of the parasite; the mosquito of the genus Anopheles, Anopheles stephensi, which is the definitive host in which the sexual or sporogonic multiplication takes place.
VII. Growth cycle :
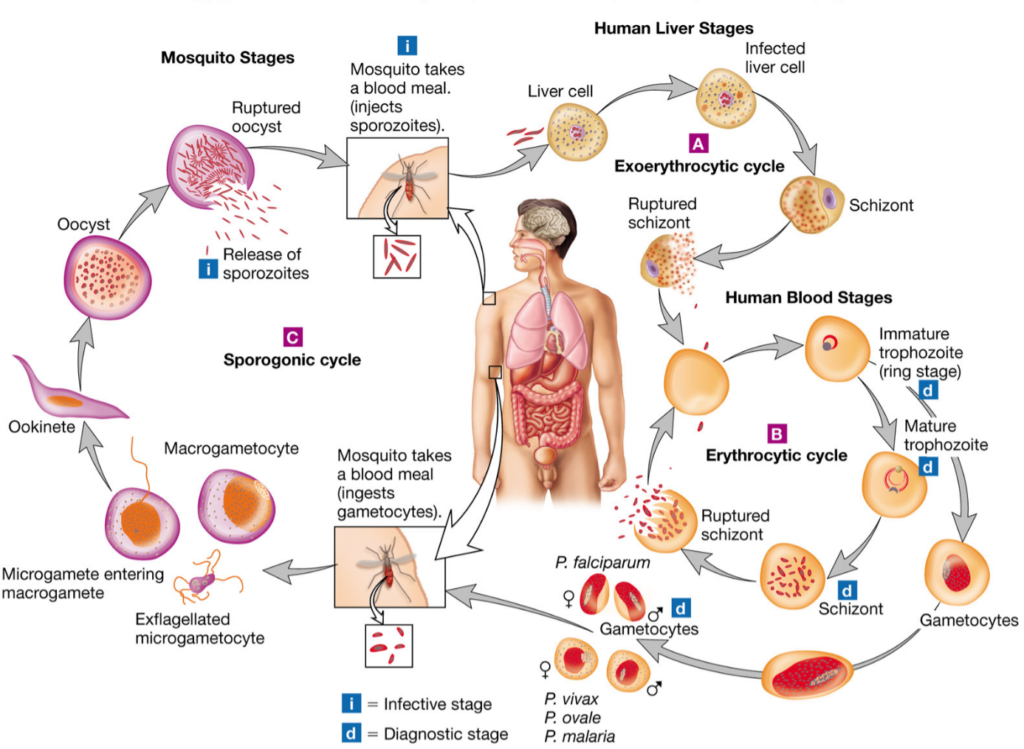
There are two phases: an asexual phase that occurs in the vertebrate host and is subdivided into two sub-phases (exo-erythrocytic phase and intra-erythrocytic phase) and a sporogonic phase that occurs in the invertebrate host (anopheles mosquito) (Figure 5).
The asexual cycle in the vertebrate host is as follows: During her blood meal, the female anopheles inoculates the human with sporozoites present in her salivary glands. These sporozoites invade the blood and rapidly reach the liver by invagination, where they begin the exo-erythrocytic phase, forming hepatic schizonts. These multiply by schizogony. This phase goes unnoticed and is asymptomatic. At the end of this phase, thousands of merozoites are generated by bursting of the hepatic schizonts. This phase may last one to two weeks depending on the species. In P. vivax and P. ovale, there is a delayed schizogony (hypnozoites) and the release of merozoites into the bloodstream can take place up to 18 months later. The erythrocytic phase begins with the invasion of red blood cells by merozoites via a ligand-receptor mechanism involving parasite proteins and red blood cell receptors (Gaur et al., 2004), thus there is a difference in host cell preference by the parasite (reticulocytes, aged red blood cells). Maturation into schizonts (2 to 3 days depending on the species), via young trophozoites (ring) and mature trophozoites, leading to the destruction of red blood cells and the release of new merozoites that infect red blood cells again. The breakdown of the red blood cells leads to fever attacks and an increase in parasitaemia characteristic of malaria attacks. For its growth, the parasite imports nutrients either from the globular cytoplasm (hemoglobin, amino acids, fatty acids, p-aminobenzoic acid, glucose) or from the globular membrane surface. It also exports proteins; some particular proteins located at the level of the red blood cell membrane promote the adhesion of parasitized and non-parasitized red blood cells in order to escape the splenic cleaning action.
When the red blood cells burst, pyrogenic substances are released as well as TNF cytokines which have a necrotizing action on the vessels and further aggravate the disease (Grau et al., 1989).
The infected erythrocyte experiences changes in structure and size, from a biconcave to a globular crenellated sphere, and its deformability is reduced. Some merozoites, after a number of cycles, are differentiated into male and female gametocytes which differ in size of the nucleus and cytoplasm and ensure the sexual cycle in the invertebrate host. Maturation of the gametocytes takes place in the human and the fertilization in the stomach of the female mosquito.
The sexual cycle in the invertebrate host is as follows: The anopheles during its blood meal inoculates the sporozoites while recovering the gametocytes from the infected vertebrate host. After 10 minutes, in its stomach, the male gametocytes transform by exflagellation and fertilize the female gametocytes to form a zygote (ookinetes). The ookinetes adhere to the stomach wall after 24 hours and pass through it, becoming oocysts. The growth of the oocysts takes 4 to 21 days, the duration of the process depending on the ambient temperature. From an initial oocyst several hundred sporozoites are formed, migrate to the salivary glands and are ready to be injected during a new puncture (Grau et al., 1989).
VIII. DIAGNOSIS
Malaria is diagnosed by two types of methods: direct and indirect. The direct ones are parasitological, while the indirect ones are based on immunology or molecular biology (Mouchet, 2004). Diagnosis is based on the detection of erythrocytic forms of plasmodium in a peripheral blood sample (Gentilini, 1993). Classically, the thick drop and the blood smear are used (Courte-joie, 2000). However, their performance in terms of sensitivity and reliability depends directly on the experience of the microscopist and the parasitemia of the infected subject (Gentilini, 1993). It is noted that some complementary examinations are to be done; we have the hemoglobin (Hb) that should be measured systematically in case of clinical anemia and severe malaria, the glycemia that should be measured systematically to detect hypoglycemia (< 3 mmol/l or < 55 mg/dl) in case of severe malaria or associated malnutrition (WHO, 2010; WHO, 2013; WHO, 2014).
IX. ENVIRONMENTAL AND PERIDOMESTIC SANITATION PRACTICES
These are preventive methods that are done either by mechanical or chemical control. Mechanical control consists of: destroying and emptying regularly objects likely to retain water such as vehicle wrecks, old tires, cans (sardines, tomatoes, etc.. Use insecticide-treated mosquito nets. Improve housing (use mosquito netting and mesh to cover windows and ventilation holes, fill in holes and cracks that are hiding places for mosquitoes); building of septic tanks; regular disposal and storage of solid waste (garbage); vegetation weeding and tree pruning; disposal of household waste in individual containers (garbage cans) or in bins placed along the streets by the administration; de-cluttering of living quarters (Fattorusso et al. , 2004). Chemical control consists of: spreading waste oil, mineral oil or diesel on the surface of stagnant water, using pesticides (insecticides with a spray machine, a fumigator…).
X. CARE PLANS
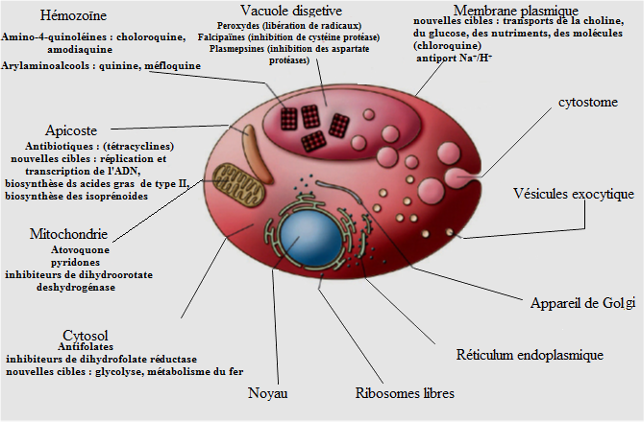
Curative treatment has two aspects: biomedicine and traditional medicine. In biomedicine, treatment involves several molecules that are classified according to their mechanisms of action as schizonticides, nucleic acid or anti-metabolite inhibitors, inhibitors of mitochondrial functions, inhibitors of protein synthesis and gametocytocides (Katzung, 2006; Njomnang, 2008). Blood schizonticides includes quinoline derivatives and artemisinin derivative. The quinoline derivatives include the four amino quinolines (Chloroquine, Amodiaquine) and the amino alcohols (Mefloquine, Halofantrine). These molecules interfere with the digestion of hemoglobin by inhibiting the formation of hemozoin. Being weak bases, these molecules concentrate in the digestive vacuoles of the parasite and act by binding to free heme and thus inhibiting heme polymerization. The detoxification process by haemozoin is blocked, resulting in the accumulation of the toxic haem for the plasmodium (Moulin and Coqueret, 2008). This mechanism is used in the present study to evaluate the antimalarial activity. Artemisinin derivatives (Artesunate, Artemether, Artemether) have a gametocytocidal action that reduces transmission and limits the chances of resistance emergence. They interfere also in the digestion of hemoglobin by releasing free radicals that are toxic for the parasites (Moulin and Coquerel, 2008). Anti-metabolics block the division of the parasite nucleus. They incorporate the anti-folics (sulfonamides) and anti-folinics (proguanil, pyrimethamine). They act by inhibiting respectively dihydrofolate synthetase and dihydrofolate reductase necessary for the biosynthesis of the parasite’s nucleic acids (Moullin and Coquerel, 2008). Mitochondrial function inhibitors are naphthoquinones (Atovaquone). The latter is a powerful inhibitor of mitochondrial functions by blocking dihydropteroate dehydrogenase. It is always used in combination with an anti-metabolite (Proguanil) to avoid the appearance of resistance (Musset, 2006). Protein synthesis inhibitors are essentially antibiotics (tetracyclines, macrolides and lyncosamides), they can inhibit the protein synthesis of the parasite; they are used in combination with other products in case of multiple resistance (Njomnang, 2008). There are also combinations, including Artemether and Lumefantrine, Artesunate and Sulfadoxine-Pyrimethamine, and Artesunate with Amodiaquine. They are recommended to limit the emergence of resistance cases. The above groups of drugs are used in the therapeutic treatment of severe and uncomplicated malaria (WHO, 2013).
In traditional medicine, antimalarial treatment uses mainly plants (N’guessan et al., 2009). In this health care system, diagnosis is based only on the symptoms of the disease, which is a source of ambiguity. The disappearance of signs, however, is evidence of the effectiveness of traditional medicines. In many countries, studies on the use of plants have confirmed their value in the treatment of malaria (Boubacar, 2005).
Several plants are currently used for that purpose. These include: Cinchona sp (Rubiaceae) bark used as a decoction, infusion or maceration; Azadirachta indica (Meliaceae) leaves as a decoction; and Artemisia annua (asteraceae) leaves as an infusion (Benoit, 1996; Njomnang, 2008). Figure 6 shows the targets of antimalarials.
XI. FOCUS ON THE DEMOCRATIC REPUBLIC CONGO (DRC) AND MALARIA
The Democratic Republic of Congo is a highly endemic country and the vast majority of the population is exposed to malaria (Figure 1). The disease is a major public health and development issue in the country. WHO statistics for 2016 show approximately 19 million cases of malaria and 42,000 related deaths. In 2007, 68% of outpatient visits recorded and 30% of hospitalizations were due to malaria, while adding that only 20% of the population attends medical centers (NMCP, 2007). The DRC is ranked after Nigeria among the most affected countries in sub-Saharan Africa (WHO, 2010). On the average, a Congolese child suffers 7-10 episodes of malaria per year. More than 27,000,000 cases of malaria are recorded each year with at least 180,000 deaths, the most affected being children under five and pregnant women (WHO, 2011).
In this large country, three species of Plasmodium are commonly encountered in malaria: P. vivax, P. malariae, and P. falciparum. Of the three, P. falciparum is the most common, accounting for 95% of cases, and it alone is responsible for the majority of morbidity and mortality. In addition, it very often presents resistance to the most commonly used and commercially available antimalarial drugs (Delacollette et al. 1983; Wilson, 1989; Mesia, 2009).
Prior to antimalarial drug resistance, chloroquine was used as a first-line drug. This resistance led to the use of the combination of sulfadoxine and pyrimetamine. A few years later, this combination encountered the same problem of resistance leading to a change in malaria management policy (Kazadi, 2003). Today, in most endemic areas, ACTs (Artemisinin Combination Therapy) are prescribed as first-line treatment for uncomplicated malaria. Quinine, which has been used for years, continues to demonstrate its efficacy and is used as monotherapy in the treatment of simple or complicated malaria in children as well as in pregnant women, both in DRC and in other endemic countries (Achan et al., 2011).
In terms of therapy in DRC, several ACTs are available: Artemisinin-derived combinations with Amodiaquine, Sulfadoxine/Pyrimethamine, Lumefantrine, Mefloquine or Piperaquine. All of these products are circulating as generics with over 60 different names. The cost of treatment does not make it accessible to everyone despite the different strategies put in place by the PNLP (National Malaria Control Program), which leads to the use of on-board resources or the return to plants in the form of raw or improved products (MTA, Improved Traditional Medicines).