Lalaina V.Rahajamanana1*, Dera S.Andriatahiana2, Paulin Andrianjakasolo2, Liliane J. Raboba1, Andry N. Ratovohery3, Andry Rasamindrakotroka2
1Department of Pediatrics, Mother and child Teaching Hospital, Antananarivo, Madagascar
2 Department of Biology, Faculty of Medicine, Antananarivo, Madagascar
3 Department of technology and information, Faculty of Medicine, Antananarivo, Madagascar
Corresponding author contact information:
Lalaina Vonintsoa RAHAJAMANANA
CHU Mère Enfant Tsaralalàna
Antananarivo-101- Madagascar
Tel: +261 (0) 34 19 321 79
E-mail address : v_lalaina@yahoo.fr
ABSTRACT
Background:
Paediatric bacterial meningitis is a major public health problem. Biological testing of CSF is the key element to confirm the disease but remains difficult to access by clinicians or patients in low-ressources settings. We describe CSF biological tests results in invasive paediatric bacterial meningitis at the University Hospital Mother and Child of Tsaralalàna (CHUMET) in Madagascar.
Methods:
From January 2013 to December 2018, all CSF samples that were confirmed for bacterial meningitis by triplex PCR Streptococcus pneumoniae, Haemophilus influenzae and Neisseria meningitides were enrolled.CSF collected from eligible childrenwere tested by microscopy, culture, soluble antigen at CHUMET laboratory. Residual CSF was referred to the Regional Reference Laboratory (RRL) for real-time polymerase chain reaction (rt-PCR) confirmatory testing and serotyping.
Results:
Over the 6-year study period, 2286 CSF were tested by PCR, 141 (6.1%) were positive. The age group of [1-12 months] was the most affected (68.0%). The majority of CSF were cloudy with pleiocytosis >100/mm3. Hyperproteinorrhea >1g/L was noted in 48.2% of cases. The sensitivity of Gram stain was respectively 56.6% and 75% for Pneumococcus and Meningococcus detection while for culture it was 28.3% and 66.6%, respectively. The average white cell count was notably higher in meningococcal meningitis and changed significantly according to the pathogens identified (p=0.007).
Conclusion:
Confirmation of the diagnosis of bacterial meningitis is based on laboratory analysis of CSF. These testing are also important for monitoring circulation of pathogens and the impact of vaccination programs.
Keywords: CSF; Haemophilus influenzae; Neisseria meningitidis; Paediatry; Streptococcus pneumoniae
INTRODUCTION
Bacterial meningitis is an invasive infection which affects with predilection children under 5 years of age. It is a medical emergency because of the risk of major neurological sequelae and even mortality if adequate management is delayed. 1It remains a public health problem in developping countries, especially in Africa, where it is associated with major sequelae and high mortality.1Some bacteria can be responsible but mainly three are reported as the most frequently incriminated in paediatrics, which are Pneumococcus or Streptococcus pneumoniae (S.pneumoniae), Meningococcus or Neisseria meningitidis (N.meningitidis) and Haemophilus influenzae type b (Hib).2 The World Health Organization (WHO) has therefore recommended the introduction of vaccines targeting those pathogens in developing countries and the establishment of an epidemiologic surveillance system to follow the trends of those bacteria.3,4If the hypothesis of meningitis was initially made on a clinical basis, the key to confirm the infection was the biological examination of the CSF. But the accessibility of these exams for patients and doctors in low-income countries is still difficult, due either to the high cost of these tests or to the lack of laboratory capacity, the majority of which don’t perform all the tests or partially.5In Madagascar, the available data on bacterial meningitis in children are mainly focused on clinical, epidemiological and therapeutical aspects.6 The aim of this study is to report the findings of cytobacteriological, chemical and molecular CSF tests in patients <5 years old with PCR-confirmed invasive bacterial meningitis.
METHODS
Study site : This study was performed at the Laboratory of the Mother and Child Teaching Hospital Tsaralalàna (CHUMET). This public 82-bed paediatric referral hospital, in the capital of Madagascar offers primarily care to local patients, although there are some patients from elsewhere in the country.This is the only WHO Vaccine Preventable Invasive Bacterial Disease (VPIBD) surveillance sentinel site in Madagascar since 2012.
Study population : Hospitalized children at CHUMET who fulfilled the WHO bacterial meningitis surveillance eligibility criteria with a CSF specimen were the study population: a child with sudden onset fever (> 38.5°C rectal or 38.0°C axillary) associated with one of the following clinical signs: neck/head stiffness, altered consciousness with no other alternative diagnosis; or with another meningeal sign.4
Surveillance circuit : CSF tests done at the sentinel site laboratory (SSL) included chemical (glucose and protein concentrations) and microbiological analyses (microscopy for cytology and Gram stain, culture and soluble antigen test for the causative organisms: Antigen detection for Streptococcus pneumoniae using AlereBinaxNOW®, antigen cards and/or the Pastorex™Meningitis Bio-Rad latex agglutination test detecting Hib, S pneumoniae, N. meningitidis groups (A, B, C, W and Y antigens), E. coli K1 and group B Streptococci.
Any residual CSF at the sentinel site laboratory was stored at -20°C and shipped to the National Institute for Communicable Diseases (NICD) Regional Reference Laboratory (RRL) in South Africa, where real-time polymerase chain reaction or PCR (rt-PCR) molecular testing was performed on all CSF samples received. Total nucleic acid (DNA) were extracted from each CSF on the MagNA pure 96 instrument (Roche) and the extracts were run on the Applied Bio-systems 7500 Fast real-time PCR instrument (Applied Biosystems, Foster City, California, USA) for the detection of ctrA, lytA, and hpd genes for confirmation of N. meningitidis, S. pneumoniae, and H. influenzae, respectively. 7
Cases were considered confirmed bacterial meningitis if any of the pathogens were detected in CSF by any of the laboratory methods.
Patient information was collected on a clinical investigation form including identity (name, date of birth, gender, and address), clinical information (diagnosis and date of admission, date of onset, previous antibiotic use, and clinical signs), vaccination status, outcome, and discharge (date and diagnosis). Laboratory test results were recorded in the Laboratory Logbook. These sentinel site clinical and laboratory data and PCR results were captured in a database.
Inclusion criteria : In this study, all children with PCR positive CSF to one of the major pathogens S. pneumoniae, Hib and N. meningitidis and analyzed by both Gram stain microscopy and/or cytology and/or biochemical examination and/or culture and/or soluble Ag tests with available results were included. The children with incomplete records were excluded.
The studied variables were patient demographic profile and laboratory test results (macroscopy, microscopy, culture, Latex soluble antigens and Binax Now S.pneumoniae).
The Chi-square test was used for statistical analysis and a p value <0.05 was considered to be significant. The performance of identification results by the routine examinations compared with the PCR results was expressed in terms of sensitivity and specificity (%).
RESULTS
A total of 4203 CSF samples from eligible children in the surveillance program were received at the CHUMET laboratory during the study period. Of these, 2286 (54.4%) were analyzed by triplex PCR for N. meningitidis, Hib and S. pneumoniae with a positive rate of 6.1% (n=141).
Children in the age group of [1-12 months[ most frequently had CSF positive for one of the three invasive bacteria, with mean age of 6 months and ranges from 0 to 59 months. The sex ratio was 1.35.
The macroscopic study showed that 43.2% (n=61) of the patients had turbid CSF whileit was clear for 6.8% of cases (n=52). Twenty-six CSF (n=26) or 18.4% were haematic.
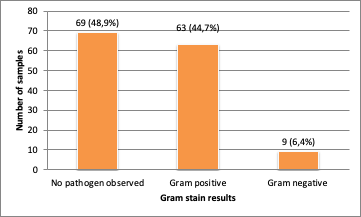
White cell counts were performed in 136 patients, 38.2% (n=52) of which had a hyperleukocytosis >100/mm3. For 36% of the patients (n=35), the lwhite cell count was <10/mm3.Of the positive CSF by PCR, Gram stain were positive in 51% of cases (n=72) (Figure1)
One hundred and thirty (n=130) CSF were analyzed for chemical tests. Protein concentration was high (>100mg/dl or >1g/l) for 52.3% of cases (n=68) while for the glucose concentration, 40.7% (n=53) of patients presented a concentration <2.20 mmol/L.
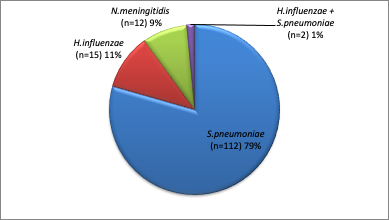
S. pneumoniae was the common pathogen identified by triplex PCR positive CSF (79%). There were two cases of co-infection with Hib and Pneumococcus. (Figure 2). The age group of [1-12 months] had the highest frequency of positive CSF, followed by the 12-24 month age group (Table 1). The mean values for white cell count, protein and glucose concentrationsby pathogen identifiedare shown in (Table 2).
Culture was done for 139 PCR positive CSF and it was positive in 28.7% of cases. It was sterile in 71.2% (n=99) of the cases. S. pneumoniae was the most frequently isolated organism in culture (23%, n=32) followed by N. meningitidis (6%, n=8). The overall sensitivity of culture for detection of Pneumococcus was 28% and 67% for Meningococcus. Of the 15 identified by PCR, no Hib was isolated in culture.
Of the 141 CSF positive by PCR, a total of 125 were tested by latex agglutination of soluble Antigen. As for culture, no Hib was identified by this test. Pneumococcus detection sensitivity was 53% and 33% for Meningococcus. Among positive CSF by PCR, 40 samples (n=40, 28.3%) were tested for S. pneumoniae soluble antigen by the BINAX NOW test (AlereR). The overall sensitivity of this test was 74.3%.
DISCUSSION
The PCR technique was selected to confirm the bacterial presence in the CSF because of its high sensitivity, even for patients with previous antibiotherapy. It detects the organism in spite of the low level of DNA, which usually limit interpretation of the routine bacteriological result (microscopic examination, culture). However, the risk of contamination is the major limitation. The PCR positivity rate was 6.1%. This differs from the findings of Goita D et al. who reported a rate of 15.2%.8The incidence of bacterial meningitis may differ by age group, terrain, country and the availability of immunization programs for vaccine preventable diseases.
The turbid appearance of the CSF was predominant in our study, which is similar to the finding of other authors.9,10,11 This aspect is related to the pleiocytosis in the CSF 12and occurs when there are 200 white blood cells/mm3, particularly in the presence of neutrophilic granulocytes. It is important for the operator to note this appearance, as it can indicate a bacterial origin of the meningitis and require the initiation of an immediate antibiotic treatment to minimize mortality risks and complications. According to Carbonelle E., approximately 10% of meningococcal meningitis may occur with normal CSF.12
The cytological analysis of the positive CSF by PCR revealed that 38.2% of patients have more than 100 leukocytes/mm3. The typical characteristic of purulent meningitis is the presence of high CSF cellularity (>500/mm³) with predominantly altered neutrophilic cells. In some cases, no white cells can be found in CSF, although bacterial inoculum is high.12 A recent study by Zimmermann P and Curtis N has reported no association between the absence of pleocytosis and the presence of specific organisms in meningitis.13 These findings suggest that absence of pleiocytosis is not a reliable exclusion of bacterial meningitis and must be interpreted in context of disease duration.
The Gram stain was positive in 51 % of cases, similar to the results of Meghraoui Y., who reported 50% positivity of cases14, but differed from Diffo C., whose results revealed 28 % positivity due toantibiotic treatment 15. A number of previous studies have reported the sensitivity of gram stain to range between 60 and 97% and specificity to around 100% without antibiotic treatment. With early treatment, sensitivity is commonly between 40% and 60%, or less.16 In our study, it was respectively 56.6% and 75% in Pneumococcus and Meningococcus detection.
The efficiency of this method depends on the amount of bacterial load in the sample, which can be reduced substantially if antibiotics are used. 17Additionally to cytological analysis, the presence of bacteria on Gram stain will confirm the bacterial meningitis and will give orientation about the involved species.
Glycorachy is not dependent on any threshold level but should be compared with blood glucose concentration and should be at least half of the latter. Nevertheless, any decrease of glycorachia under 2.20mmol/L would suggest a pyogenic or tuberculosis meningitis rather than viral etiology. However, low levels of glucose concentration are not specific of bacterial infections since it can be found in other situations (inherited metabolic diseases.18 And when it is too low, it indicates a bad outcome.
Culture positivity rate in this study was higher than the finding of Malki19 and Carlyse D15but lower than Bouskraoui M et al results20in 2014 where culture was positive in 54.5% of cases.
These differences can be related to a prior antibiotic administration before lumbar puncture and to the inadequate pre analytical conditions of CSF which can lead to the negativation of culture despite the infection14These invasive organisms are very sensitive to extreme temperature fluctuations. A very low bacterial inoculum could also explain the culture negativity. According to a recent study, a prior antibiotherapy reduced the positivity rate of the CSF cultures from 95 to 68%.21
This study found that culture was more sensitive in identifying meningococcus than pneumococcus (28.3% vs. 66.6%). Prior antibiotic treatment may strongly impact the culture. In Madagascar, amoxicillin or ampicillin are widely available in ambulatory practice, sometimes as self-medication by the parents. S. pneumoniae is more susceptible to these antibiotics while for N. meningitidis, reduced susceptibility to Penicillin G and aminopenicillins is more common and has been reported. The high sensitivity of PCR enables the amplification of the DNA, although bacterial growth may be inhibited by the antibiotics, which explains the findings in this study. No Hib was isolated in culture among the fifteen identified by PCR. Nutritional requirement of the species and the use of antibiotic prior to hospitalization could explain this result.
Despite low sensitivity of the culture, this method remains the gold standard for the diagnosis of bacterial meningitis14 and allows antibiotic susceptibility testing and antimicrobial resistance monitoring.
The agglutination test for soluble antigen detection has low sensitivity, and for meningococcus, performance of agglutination reagents depends on the serogroup involved.22Although the BINAX S. pneumoniaetest was used in only 28.3% of the CSF tested, its sensitivity in detecting the pathogen was better than the other bacteriologic test (direct examination, culture, latex soluble ag). This rapid diagnostic test, easy to use and requiring no special equipment, is advantageous in the regional healthcare structures where the laboratory’s capacity to perform complete microbiological tests is often lacking. This test offers possibilityto improve patients care and it should be used as a complementary tool to microscopic and biochemistry tests.
New vaccine introduction into vaccination program of Ministry of Health for many country has changed the epidemiology of bacterial meningitis. H. influenzae type b meningitis has practically disappeared since the introduction of the Hib vaccine in routine immunizations.Several studies in African countries have reported a predominance of S. pneumoniae. 8, 14, 15, 23
Meningococcus represent 8.5% of PCR identified pathogens. It is the only bacterial meningitis that can cause epidemics. For a country, an epidemic situation can be defined by an unacceptable incidence rate requiring action.
This study was limited by being conducted in a single paediatric Hospital of Madagascar and might not reflect the trends and the situation in other facilities. But it highlighted the role of the laboratory tests to identify pathogens mostly associated with paediatric bacterial meningitis in a low-resource country. A variety of biological tests are actually made available to confirm invasive pathogens in CSF. These assays are complimentary and must be optimally used to improve the early management of these infection and to avoid severe complications for the patient. The CSF biological tests are important for paediatric bacterial meningitis diagnosis and for monitoring the trends of invasive bacteria as effectiveness of vaccination programs.
ACKNOWLEDGMENT
We thank the following people:
- Medical Staff, medical trainee, staff in laboratory from CHUMET, Antananarivo
- World Health Organization
- Laboratory staff from National Institute for Communicable Disease of the National Health Laboratory Service in Johannesburg, South Africa
DECLARATIONS
Funding: None
Conflict of interest: None declared
Ethical approval: Not required
REFERENCES
- Bosdure E. Méningites bactériennes de l’enfant et complications.3èmeéditions .Flammarion ; Neurologie Pédiatrique.2010 : 403-17
- Djeungoue SJ. Epidémiologie de la méningite bactérienne auMali [Thèse].Pédiatrie : Bamako; 2008. 87p.
- World Health Organization. Pneumococcal conjugate vaccine for childhood immunization-WHO position paper. Weekly Epidemiological Record, 2007 : 82 (12), 93 – 104. Availableat https://apps.who.int/iris/handle/10665/240901. Accessed 20 April 2022
- World Health Organization. WHO vaccine-preventable diseases surveillance standards. 2018. Available at:https://www.who.int/immunization/monitoring_surveillance/burden/vpd/standards/en. Accessed 20 April 2022
- WHO.INT. Surveillance de la méningite épidémique dans la ceinture africaine.OMS 2014 Available at : https://apps.who.int/iris/bitstream/handle/10665/135936/WHO_HSE_PED_CED_14.1_fre.pdf?sequence=1. Accessed 19 december 2021
- Mioramalala S.A, Razafindratovo RMR, Rakotozanany A, Raharizo M, Weldegebriel G, Mwenda et al.Analysis of Death and Survival Factors Associated withChildhood Bacterial Meningitis at a Reference Pediatric Hospital in Antananarivo, MadagascarImmunol Sci. 2018 July 2; Suppl(2): 8–14.
- Wang X, Theodore MJ, delMair R, et al. Clinical Validation of Multiplex Real-Time PCR Assays for Detectionof Bacterial Meningitis Pathogens.JClinMicrobiol 2012; 50(3):702.
- Dao S, Goita D, Oumar Aa, Diarra S, Traore S, Bougoudogo F. Aspects épidémiologiques des méningites purulentes au Mali. Médecine d’Afrique Noire. 2008;55:515-8.
- Lewagalu V, Tikoduadua L, Azzopardi K, Seduadua A., Temple B, Richmond P.et al. Meningitis in children in Fiji: etiology, epidemiology, and neurological sequelae.Int J Infect Dis. 2012; 16: 289–954
- Diarra F. Facteurs pronostiques et devenir des enfants atteints de méningite bactérienne dans le département de pédiatrie du CHU Gabriel Toure [Thèse].Pédiatrie: Mali ; 2012. 98p
- Rafaravavy NE.Aspectsépidémio-cliniques et bactériologiques de la méningite de l’enfant hospitalisé à l’HUMET [Mémoire].Pédiatrie : Antananarivo;2012.40p.
- Carbonnelle E. Apport des examens biologiques dans le diagnostic positif, la détermination de l’étiologie et le suivi d’une méningite suspectée bactérienne. Masson; 2009; 39: 581-605
- Zimmermann P,Curtis N. Bacterial meningitis in the absence of pleocytosis in children. Pediatr Infect Dis J. 2021 Jun 1;40(6):582-7.
- Meghraoui Y. Les méningites bactériennes au service de pédiatrie du CHU Mohammed VI [Thèse] .Pédiatrie : Marrackech;2018. 91p
- Carlyse DS. Epidémiologie des méningites à l’hôpital mère-enfants Marrakech. [Thèse].Pédiatrie : Marrakech; 2013. 197p.
- Neuman M, Tolford S, Harpe MB . Test characteristics and interpretation of cerebrospinal fluid Gram stain in children.Pediatr Infect Dis. 2008 Apr;27(4):309-13
- Alonso JM., Taha MK. Respiratory virosis and invasive bacterial super infections: the case for influenzae and meningococcal diseases. Arch Pediatr 2003; 10 : 1013-5.
- Carbonnelle E. Apport des examens biologiques dans le diagnostic positif, la détermination de l’étiologie et le suivi d’une méningite suspectée bactérienne. Masson; 2009; 39: 581-605
- MalkiM. Les méningites purulentes du nourrisson et de l’enfant à propos de 49 cas [Thèse].Pédiatrie:Fès; 2008.187p
- Bouskraoui M,Bourrous M, Azher A. Les méningites purulentes de l’enfant : Epidémiologie,diagnostic, traitementetprévention. L’Antibiothérapie en pédiatrie. 2010
- Roine I, Foncea LM, Ledermann W, Peltola H. Slow recovery of cerebrospinal fluid glucose and protein concentrations distinguish pneumococcal from Haemophilus influenzae and meningococcal meningitis in children. Pediatr Infect Dis J. 1995;14:905–7
- Cavallo JD,Nicolas P,Martet G. Actualités sur la sensibilité de Neisseria meningitidis aux antibiotiques et en particulier aux bêtalactamines. La Lettre de l’Infectiologue.1998;9 :429-33
.
- Société Marocaine d’Infectiologie Pédiatrique et de Vaccinologie. Recommandations pratiques pour la prise en charge des méningites bactériennes aiguës de l’enfant au Maroc. SOMIPEV; 2014. Available at https://somipev.ma/fr/component/edocman/recommandations-pratiques-pour-la-prise-en-charge-des-m%C3%A9ningites-bact%C3%A9riennes-aigu%C3%ABs-de-l%E2%80%99enfant-au-maroc.html. Accessed 20 April 2022
| PCR RESULTS | AGE GROUP (month) | |||
| [0- 1[ (n) | [12-24[ (n) | [12-24[ (n) | [24 – 59 [ (n) | |
| S.pneumoniae | 6 | 81 | 15 | 10 |
| H.influenzae | 3 | 8 | 4 | 0 |
| N.meningitidis | 2 | 7 | 2 | 1 |
| S.pneumoniae + H influenzae | 0 | 0 | 2 | 0 |
| Total | 11 | 96 | 23 | 11 |
| PCR RESULTS | MEAN | ||
| White cell count (/mm3) [min – max] | Proteinorrachia (g/L) [min – max] | Glycorachia (mmol/L) [min – max] | |
| S.pneumoniae | 442 [0-1500] | 1,68 [0,01-6,17] | 2,47 [0,01-12,63] |
| H.influenzae | 226 [0-9000] | 1,48 [0,18-6,12] | 3,20 [1,01-5,79] |
| N.meningitidis | 874 [0-4500] | 2,82 [1,34-4,65] | 1,65 [0,01-7,49] |
| p-value | 0,007 | 0,108 | 0,132 |
Min : Minimum ; Max : Maximum