Lalaina V.Rahajamanana1,2*, Paulin Andrianjakasolo2, Dera S.Andriatahiana2, Zakasoa Ravaoarisaina3, Liliane J. Raboba1, Andry Rasamindrakotroka2
1Department of Pediatrics, Mother and child Teaching Hospital Tsaralalàna, Antananarivo, Madagascar
2 Department of Biology, Faculty of Medicine, Antananarivo, Madagascar
3Department of Pediatrics, Mother and child Teaching Hospital,Ambohimiandra Antananarivo, Madagascar
*Corresponding author contact information:
Lalaina Vonintsoa RAHAJAMANANA
CHU Mère Enfant Tsaralalàna
Antananarivo-101- Madagascar
Tel: +26134 19 321 79
E-mail address : v_lalaina@yahoo.fr
Co-authors email address
Paulin Andrianjakasolo : docteurrazanakolona@gmail.com
Dera S.Andriatahiana : deraandriatahiana@gmail.com
Zakasoa Ravaoarisaina : zakasoa.ravaoarisaina@yahoo.com
Liliane J. Raboba : liliane_raboba@yahoo.fr
Andry Rasamindrakotroka : andryrasamindrakotroka@idriss-tchana
ABSTRACT
Background: Diarrheal diseases are a major public health problem in developing countries with high mortality and morbidity rates, especially among children. Shigella sp is the leading cause of pediatric bacterial diarrhea and shigellosis data are very scarce in Madagascar.
Material and methods: A 4-year retrospective study, from January 2018 to December 2020, at the University Hospital Mother and Child Tsaralalàna laboratory was performed to assess the bacteriological and epidemiological characteristics of laboratory confirmed shigellosis cases
Results: During the study period, 223 stool samples were examined, of which 45 (20.7%) were positive for Shigella sp. The mean age of infected children was 29.8 months, predominantly in the 24-59 month age group. The infection was found mainly in male children (54.3%). Most isolates of Shigella sp showed resistance to co-trimoxazole and amoxicillin. All the strains were susceptible to third-generation cephalosporins. Of the isolated Shigella sp, 14 strains were tested for species identification and serotyping, twelve of which were Shigella flexneri and two were Shigella sonnei. The most frequent serotypes were Shigella flexneri 1b and 2a.
Conclusion: This study found a Shigella sp positivity rate of 20.7%. This pathogen frequently infects infants age group. Bacteriology laboratory surveillance and a multicenter survey are essential to control the spread of drug-resistant Shigella and to monitor circulating strains and the burden of this disease. Awareness of water, hygiene, and sanitation (WASH) and community water supply is also necessary to reduce this infection.
Keywords: antibiotic, laboratory, Paediatry, serotype, Shigella,
INTRODUCTION
Shigellosis or bacillary dysentery is a bacterial diarrhea due to bacteria of the genus Shigella sp1. The relatively high morbidity affects mostly children under 5 years of age. 2 The clinical symptoms are related to the invasive nature of Shigella sp on the intestinal epithelium associated with a strong inflammatory reaction in the lamina propria. Additionally to this invasive mechanism, toxin secretion leads to intestinal hypersecretion. These physiopathological mechanisms result clinically in a dysenteric syndrom with bloody stools, abdominal pain, tenesma and fever. 4 In older children and young people, the disease can be mildly symptomatic. 5,6 The diagnosis of shigellosis is based on the laboratory identification of Shigella by bacteriological examination. Coproculture remains the gold standard in the laboratory. Rarely, a blood culture is requested to diagnose shigellosis. Other biological tests, like molecular biology, are currently available for the detection of Shigella sp but they do not allow antibiotic susceptibility testing. The Shigella genus contains 4 species: Shigella flexneri, Shigella boydii, Shigella dysenteriae and Shigella sonnei, which are classified into several serotypes according to the biochemical and antigenic characteristics of the bacteria.7 Shigella sp is the second leading cause of death from infectious diarrhea in children after rotavirus and is the leading cause of bacterial diarrhea.8 Furthermore, shigellosis in the past was very different from the current situation. In the past, Shigella dysenteriae was the commonest species responsible for severe disease and it has been replaced by Shigella flexneri in many countries.9,10 The treatment is based on antibiotic therapy.11
Also the emergence of multidrug-resistant bacteria is threatening worldwide due to the irrational use of antibiotics both in developed and developing countries12, so that the WHO established a global antimicrobial resistance surveillance to tackle these new problems and Shigella sp is one of its target pathogens. Data on shigellosis in Madagascar are very scarce. This study aims to describe the epidemiological and bacteriological profile of Shigella sp strains circulating in Antananarivo Madagascar.
MATERIALS AND METHOD
It was a 3-year descriptive retrospective study, from January 2018 to December 2020, performed at the Mother and Child University Hospital of Tsaralalana (CHUMET) bacteriology laboratory. This 82-bed public pediatric referral hospital in the capital of Madagascar offers care primarily to local patients under 15 years of age, although there are some patients from elsewhere in the country.
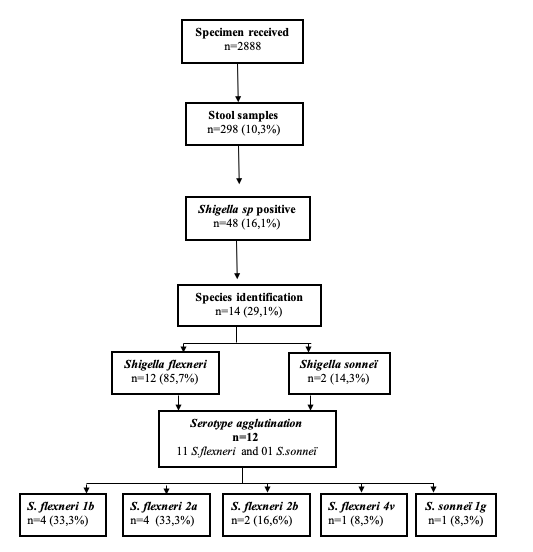
All hospitalized and outpatient children who performed a stool culture at the CHUMET laboratory with a positive result for Shigella sp with all available results were included. Coprocultures positive for other pathogens were excluded. The laboratory logbook and patient records were used to collect data and the analysis was performed with EPI info v7.0. The studied variables were: socio-demographic characteristics (age, gender, origin of the sample), bacteriological results (species and serotypes of Shigella sp isolated, susceptibility profile to antibiotics routinely used in practice). Stool samples were plated on a selective culture medium Hektoen agar (Biokar Diagnostics, Allonne, France). A phenotypic method by the appearance of colonies was used for identification. The appearance of suspect colonies (lactose negative) on this medium was followed by the isolation of three isolated colonies on another Hektoen agar and simultaneously on a chromogenic agar Uriselect (BioRad, Californie, Etats – Unis). Lactose-negative colonies on Hektoen agar with a small white appearance on chromogenic agar were further tested by biochemical identification using the API 20E gallery (BioMérieux, Marcy l’Etoile, France). Antibiotics susceptibility testing of Shigella sp. were performed by Kirby Bauer disc diffusion method according to the current CASFM/EUCAST standard. The antibiotics tested were amoxicillin, amoxicillin/ clavulanic acid, ceftriaxon, Imipenem, gentamicin, amikacin, ciprofloxacin, and sulfamethoxazole/trimethoprim. All the Shigella sp isolated strains were stored in a 20% glycerol – Brain Infusion (BHI) storage medium at -80°C. A total of 14 Shigella sp strains were tested for serological identification or serotype by slide agglutination according to the manufacturer’s instructions (Shigella anti-sera, Eurobio scientific®, France) (Figure 1) which enabled identification of Shigella dysenteriae, flexneri, sonnei or bodyii species and serotypes of each. The test kit includes ready-to-use polyvalent and monovalent sera for the determination of serotypes belonging to each group.
RESULTS
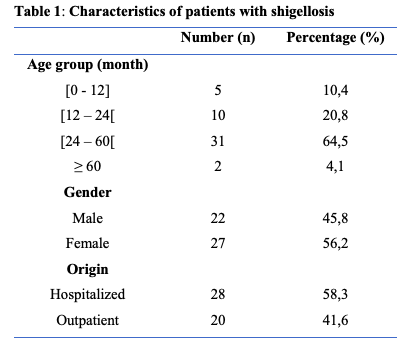
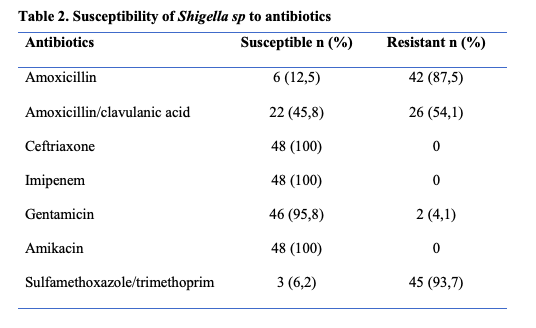
During the study period, 298 stool specimens from patients under the age of 15 years were studied, of which 48 (16.1%) were positive for Shigella sp (Figure 1). The ages of the Shigella patients range from 8 to 96 months with a mean age of 29.7 months. The peak frequency was in the age range 24 – 60 months (Table 1). The sex ratio was 1.1. Twenty-eight (n=28, 58.3%) stool cultures were from hospitalized patients. Shigellosis cases in this study were all community acquired infections. The Kirby-Bauer disc diffusion method showed marked drug resistance of the Shigella sp strains to cotrimoxazole (93.7%), aminopenicillin (87.5%), amoxicillin-clavulanic acid (54.1%) (Table 2). No extended-spectrum beta-lactamase (ESBL)-producing strains were found. Fourteen Shigella sp strains were identified by species in this study, with a predominance of Shigella flexneri (n=12, 85.7%) and Shigella sonnei (n=2, 14.3%). (Figure 1)
DISCUSSION
Shigellosis or bacillary dysentery is a fecal peril disease caused by Enterobacteriaceae of the genus Shigella sp. This disease has been responsible for important epidemics in wartime. They currently persist in endemic form in tropical countries, where they occur frequently, particularly during the hot, wet season of the year.13 During this study, two hundred and ninety-eight coprocultures (n=298) were performed in the bacteriology laboratory of CHUMET, which represented 10.1% of all samples received in the laboratory. Forty-five (n=45) were positive for Shigella sp, giving a positivity rate of 16.1%. This finding was lower compared with other studies in resource-limited countries like Nepal and Togo where the positivity rate was 52.2%, 47% respectively. 14,15,16
This rate may be certainly underestimated, since coproculture is not routinely performed in all dysenteric syndromes, due mainly to the accessibility of this test in public hospitals, as well as to the high cost of the assay and to the lack of good laboratory capacity. The conventional bacterial culture remains the gold standard for the biological diagnosis of shigellosis. It also allows for antibiotic susceptibility testing, which is particularly important in this era of antimicrobial resistance. The mean age of the children in our study was 29.7 months, with range of 8 and 96 months. Most of the children were less than 60 months of age (95.8%), with high frequency of positivity in the 24-59 month age range. Our results are consistent with other studies from 2017 and 2018 that reported a high frequency of shigellosis in this age group. 16,17 The vulnerability of children under 5 years of age to shigellosis might be due to their dietary behavior as well as a problem of sanitation and accessibility to safe water. According to the literature, children under 5 years of age are the principal targets of shigellosis and they are rarely infected before the age of 6 months if they are breast-fed.12,18
We found the predominance of males. This result was similar to other published studies reporting a male predominance. 15, 16, 19 Genetics factors could explain this male over female infectious predominance.
Shigellosis is a highly infectious disease. 20, 21 The most common symptom of shigellosis is the dysenteric syndrome, which is manifested by afecal, frequent, glairy, bloody, and sometimes mucopurulent stools, abdominal pain, epithelial discharge, tenesmus with false needs. The majority of the patients with shigellosis were hospitalized which was also found by other authors.14,22
The clinical manifestations of shigellosis may vary in different degree, they can be well tolerated by patients but they can lead to hospitalization because of complications which can be immediate or delayed, like dehydration with hydroelectric losses, neurological damage (convulsions, consciousness disorders), hemolytic uremic syndrome (HUS) and severe malnutrition. 12,18
In contrast to other diarrhoeal diseases, the therapy for shigellosis cannot be treated by rehydration alone. The first-line treatment is based on antibiotics, which generally allow a rapid recovery without sequelae. 11
The classical therapy for Shigellosis consisted of the use of aminopenicillin- amoxicillin or cotrimoxazole, but since several years, a spread of drug-resistant strains has been reported. 12, 23 Monitoring of disease incidence and the antimicrobial susceptibility of the strains are important for appropriate curative treatment and patient management. Acquired resistance was noted in this study with a high proportion of resistant strains to sulfamethoxazole/trimethoprim, amoxicillin and amoxicillin and clavulanic acid. On the other hand, all strains were susceptible to third-generation cephalosporin and imipenem, 95.7% and 91.3% to gentamicin and ciprofloxacin, respectively. Multiple drug resistance was observed in more than two-thirds of Shigella isolates. Lango-Yaya et al in 2017 from the Central African Republic reported a high level of amino penicillin and co-trimoxazole resistance of about 100%. 24 Shigella sp is group 0 in the beta-lactam susceptibility phenotypic classification. They are naturally susceptible to all beta-lactams and antibiotics used in practice. During the last half century, an alarming increase in antimicrobial resistance has been reported, especially in developing countries, where use of these medications is relatively limited. In fact, the extraordinary ability of Shigella to acquire plasmid-encoded resistance to antimicrobial drugs previously considered as first-line treatments has been demonstrated. 25 The spread of bacterial resistance is due to the irrational overuse of antibiotics in veterinary medicine. The use of azithromycin is currently recommended for the treatment of shigellosis 28 but this antibiotic was not tested in our study due to the non-availability of the disc from local suppliers. The frequency of the different Shigella species varies in different parts of the world. 27,28 People living in resource limited countries have natural immunity to Shigella sonnei from exposure to feces contaminated water containing Pleisomonas shigelloid O17 29,30 which has a
similar O type antigen as this strain. Also, Shigella sonnei is phagocytized by the Acanthomoebeoe casthalanni, ubiquitous amoeba that phagocytizes S sonnei in nature and provides an intracellular environment immune to the use of chlorination and other forms of sanitation processes. In contrast, S flexneri is lethal to the amoeba A. castellanii and cannot have this protection. 31,32 Despite the numbers studied strains, Shigella serotypes isolated were Shigella flexneri 1b and Shigella flexneri 2a. Shigella sonnei serotype g. Knowledge of the serotype has no immediately impact on the management of the patient. However, it is critical for the monitoring of Shigella virulence, for epidemiologic surveillance and for vaccine development.
CONCLUSION Shigellosis or bacillary dysentery is a public health problem with a high morbidity in the developing countries. Despite the limits of the number of sites and the number of tested strains in this study, it showed a picture of Shigella species and serotypes circulating and the antibiotic susceptibility for treatment in Madagascar. Bacteriology laboratory has a crucial role in diagnosis and treatment of shigellosis cases as well as in epidemiological surveillance which needs to be ongoing and extend to other parts of the country.
ACKNOWLEDGMENT
We thank the following people :
- Medical Staff, medical trainee, staff laboratory from CHUMET, Antananarivo
- Doctor Collard Jean Marc, microbiologist
DECLARATIONS
Funding : None
Conflict of interest : None declared
Ethical approval : Not required
REFERENCES
1. Nicolas X, Granier H, Le Guen P. Shigellose ou dysenterie bacillaire. Presse Médicale. 2007;36(11, Part 2):1606‑18.
2. Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. The Lancet. 2013;382 (9888) :209‑22.
3. Kazi A, Hisyam Ismail CMK, Anthony AA, Chuah C, Leow CH, Lim BH, et al. Designing and evaluation of an antibody-targeted chimeric recombinant vaccine encoding Shigella flexneri outer membrane antigens. Infection, Genetics and Evolution: Journal of Molecular Epidemiology and Evolutionary Genetics in Infectious Diseases. 2020;80:104176.
4. Miron D, Sochotnick I, Yardeni D, Kawar B, Siplovich L. Surgical complications of shigellosis in children. The Pediatric Infectious Disease Journal. 2000;19(9):898–900.
5. Cruz CBN, Souza MCS , Serra PT, Santos I, Balieiro A, Pieri FA, et al. Virulence Factors Associated with Pediatric Shigellosis in Brazilian Amazon. Vol. 2014, BioMed Research International. Hindawi; 2014 [Accessed 2020 july 05] avalaible from :https://www.hindawi.com/journals/bmri/2014/539697
6. Sangeetha AV, Parija SC, Mandal J, Krishnamurthy S. Clinical and Microbiological Profiles of Shigellosis in Children. Journal of Health, Population, and Nutrition. 2014;32(4):580‑6.
7. Gentle A, Ashton PM, Dallman TJ, Jenkins C. Evaluation of Molecular Methods for Serotyping Shigella flexneri. Journal of Clinical Microbiology.2016;54(6):1456‑61
.
8. Williams PCM, Berkley JA. Guidelines for the treatment of dysentery (shigellosis): a systematic review of the evidence. Paediatrics and International Child Health. 2018;38(sup1):S50‑65.
9. Seidlein L von, Kim DR, Ali M, Lee H, Wang X, Thiem VD, et al. A Multicentre Study of Shigella Diarrhoea in Six Asian Countries: Disease Burden, Clinical Manifestations, and Microbiology. PLOS Medicine.2006;3(9):353.DOI: 10.1371/ journal.pmed.0030353
10. S, Strockbine NA, Panchalingam S, Tennant SM, Barry EM, Marohn ME, et al. Shigella Isolates From the Global Enteric Multicenter Study Inform Vaccine Development. Clinical Infectious Disease.2014;59(7):933‑41.
11.Soraa N, Zerouali K. Shigella : Données épidémiologiques et Recommandations nationales. Société Marocaine d’Infectiologie Pédiatrique et de Vaccination (SOMIPEV) 2020. [Accessed 2020 January 01] Available from : http://www.somfipev.ma/fr/publications/publications-marocaines/101-publications-marocaines/278-shigella-donné-épidémiologiques-et-recommandations-nationales.html.
12. Ministère de la Santé et des Services sociaux du Québec. Maladies infectieuses: Shigellose. 2016. [Accessed January 2020] Available from https://publications.msss.gouv.qc.ca/msss/fichiers/guide-garderie/chap7-shigellose.pdf.
13. Camacho AI, Irache JM, Gamazo C. Recent progress towards development of a Shigella vaccine. Expert Review of Vaccines.2013;12(1):43‑55.
14. Ansari S, Sherchand JB, Parajuli K, Mishra SK, Dahal RK, Shrestha S, et al. Bacterial etiology of acute diarrhea in children under five years of age. Journal of Nepal Health Research Council.2012;10(22):218‑23.
15. Dossim, A. Dagnra, M. Salou, D.K. Ekouevi, Y. T. Nyasenu, S. Tigossou and M. Prince-David.Etiologies des diarrhees infectieuses chez les enfants de moins de cinq ans (5 ans) au Centre Hospitalier Universitaire Sylvanus Olympio de Lome de 2005 à 2009.Revue Bio-Africa.2014;13:32-36
16. Ateudjieu J, Beyala Bita’a L, Guenou E, Njimbia Chebe A, Chukuwchindun BA, Goura AP, et al. Profil et antibiosensibilité des bactéries pathogènes associées aux diarrhées chez les patients consultant à l’Hôpital Régional Annexe de Kousseri, Extrême-Nord Cameroun. Pan African Medical Journal.2018;29:170-182.
17. Wondwossen A, Alemu E, Solomon T, Mesfin A, Adane E and Girma G. Prevalence and antibiotic susceptibility patterns of Shigella and Salmonella among children aged below five years with Diarrhoea attending Nigist Eleni Mohammed memorial hospital, South Ethiopia. BMC Pediatrics. 2018; 18(1):241.
18. Aubry P, Gaüzère BA. Shigelloses : Actualités 2016.MedicineTropicale. 2016. [Accessed 2020 January 02]Available from http://medecinetropicale.free.fr/cours/shigellose.pdf. [Accessed 24 January 2020]
19. Sah SK, Basnet S, Shrestha S, Ghale K, Tamang S, Mandal DK, et al. Burden of Shigella spp and Vibrio spp, and their antibiotic sensitivity pattern in the patients with acute gastroenteritis in tertiary care hospital in Nepal. BMC Research Notes. 2019;12(1):699-704.
20 Dekker J, Frank K. Salmonella, Shigella, and Yersinia. Clinics in laboratorymedicine.2015;35(2):225‑46.
21. Liu J, Platts-Mills JA, Juma J, Kabir F, Nkeze J, Okoi C, et al. Use of quantitative molecular diagnostic methods to identify causes of diarrhoea in children: a reanalysis of the GEMS case-control study. The Lancet.2016;388(10051):1291‑301.
22. Temu MM, Kaatano GM, Miyaye ND, Buhalata SN, Shushu M-L, Kishamawe C et al. Antimicrobial susceptibility of Shigella flexneri and S. dysenteriae isolated from stool specimens of patients with bloody diarrhoea in Mwanza, Tanzania. Tanzania Health Research Bulletin. 2007;9(3):186-9
23. Daudens E, Dejour-Salamanca D, Isnard H, Mariani-Kurkdjian P, Filliol I, Bingen E. Épidémie de gastro-entérites aiguës à Shigella sonnei résistantes à l’amoxicilline, au cotrimoxazole et à l’azithromycine en Île-de-France Janvier – Avril 2007. Institut de Veille Sanitaire. Maladies Infectieuses. 2009. 21 p
24. Yaya EL, Djeintote M, Djimeli CL, kpinde CM, Nambei WS, et al.Contribution to the Study of Antibiotic Resistance on Salmonella and Shigella Strains Isolated in Central African Republic. Journal ofl Microbiology&Experimentation.4(1):00105.DOI:0.15406/jmen.2017.04.00105
25. Niyogi SK. Shigellosis. Journal of Microbiology.2005;43(2):133‑43.
26. Pourakbari B, Mamishi S, Mashoori N, Mahboobi N, Ashtiani MH, Afsharpaiman S et al. Frequency and antimicrobial susceptibility of Shigella species isolated in Children Medical Center Hospital, Tehran, Iran, 2001-2006. The Brazilian Journal of Infectious Diseases. 2010;14(2):153-7.
27. Ranjbar R, Bolandian M, Behzadi P. Virulotyping of Shigella spp. isolated from pediatric patients in Tehran, Iran. Acta Microbiologica et Immunologica Hungarica.2017;64(1):71‑80.
28. Sousa MÂB, Mendes EN, Collares GB, Péret-Filho LA, Penna FJ, Magalhães PP. Shigella in Brazilian children with acute diarrhoea: prevalence, antimicrobial resistance and virulence genes. Memórias do Instituto Oswaldo Cruz.2013;108(1):30‑5.
29. Sack DA, Hoque S, Etheridge M, Huq A. Is protection against shigellosis induced by natural infection with Plesiomonas shigelloides? The Lancet.1994;343(8910):1413‑5.
30. Shepherd JG, Wang L, Reeves PR. Comparison of O-Antigen Gene Clusters of Escherichia coli (Shigella) Sonnei and Plesiomonas shigelloides O17: Sonnei Gained Its Current Plasmid-Borne O-Antigen Genes from P. shigelloides in a Recent Event.
InfectionandImmunity.2000;68(10):6056‑61.
31. Kotloff KL, Riddle MS, Platts-Mills JA, Pavlinac P, Zaidi AKM. Shigellosis.TheLancet.2018;391(10122):801‑12.
32. Jeong HJ, Jang ES, Han BI, Lee KH, Ock MS, Kong HH, et al. Acanthamoeba: Could it be an environmental host of Shigella? Experimental parasitology.2007;115(2):181‑6.